Atmospheric CO2 Increase Is Not From Ocean Outgassing
(Adapted from a blog comment.)
June 12, 2020. Last updated Dec. 15, 2024.
Loydo wrote, "You’re 'told' that the CO2 must be from warmer oceans out-gassing… you’ve been gaslighted if you believe that. The ocean is a net CO2 sink..."
Exactly correct, Loydo.
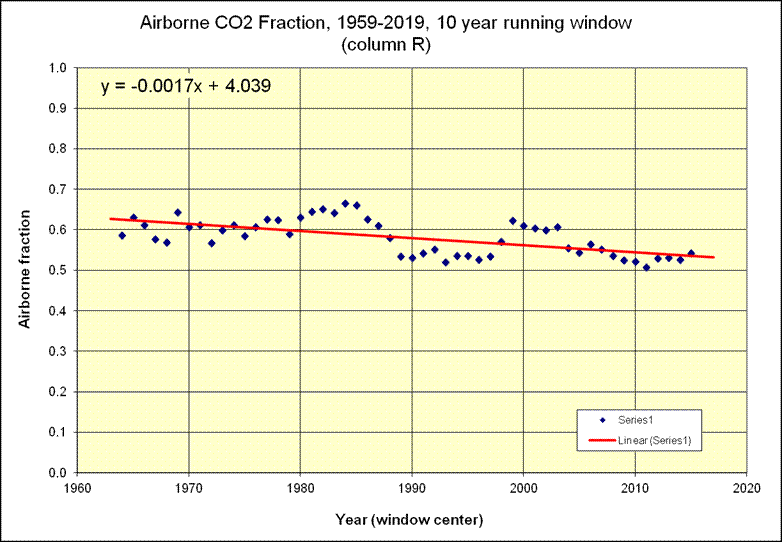
Mankind is currently adding about 5 ppmv of CO2 (about 10½ PgC) to the atmosphere each year, but the atmospheric CO2 level is only rising at a rate of about 2.5 ppmv per year. The difference is the rate at which natural negative feedbacks (mainly terrestrial greening and absorption by the oceans) remove CO2 from the air: currently about 2.5 ppmv per year. Someone who claims nature is raising the atmospheric CO2 level must be incapable of subtracting 2.5 from 5.
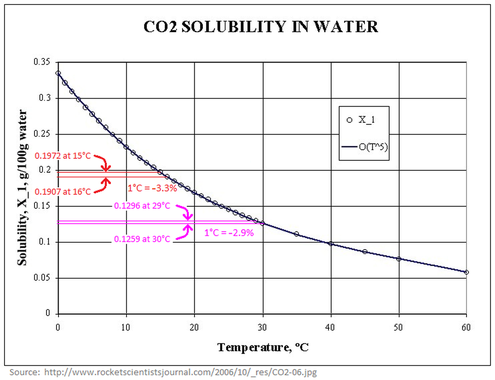
However, the solubility of gases like CO2 (or CH4) in water does decrease as the water gets warmer (per the temperature dependence of Henry's law), so as the oceans warm they would outgas CO2, if nothing else changed. The capacity of the water to hold dissolved CO2 decreases by about 3-4% per 1°C by which the water warms.
So, when the oceans are absorbing CO2, as is currently the case in most places other than the tropics, if the water warms then the oceans absorb CO2 slightly more slowly.
The effect of temperature change on the solubility of gases in water is surely one of the reasons that atmospheric CO2 levels swing up & down by about 90 ppmv over glaciation/deglaciation cycles. (There are almost certainly also biological [2] and/or ice sheet burial mechanisms at work, which increase the magnitude of glacial-interglacial CO2 swings.)
The CO2, in turn, works as a GHG to cause warming. That is a slight positive feedback mechanism.
That positive feedback loop is undoubtedly one of the causes for the apparent hysteresis [2] in the temperature and CO2 records: Over the last million years, the Earth's climate has tended to be either mild, as in our current interglacial (the Holocene), or, more of the time, heavily glaciated and cold, with relatively brief, unstable transitions between. (But see also: Deglaciation / Volcanism / CO2 Feedback.)
In paleoclimate reconstructions from ice cores, CO2 level changes generally lag temperature changes by hundreds of years, which is consistent with the fact that higher CO2 levels not only cause higher temperatures, but are also caused by higher ocean temperatures, and ocean temperature is slow to respond to air temperature changes.
Note that the rate at which the ocean absorbs CO2 from the air is proportional to CO2's partial pressure in the air. That's intuitively obvious when you remember that the concentration of CO2 in the air determines the rate at which CO2 molecules collide with and are absorbed by the surface of the ocean, and falling raindrops. So the measly 3-4% per °C, by which CO2 solubility in water decreases as the water warms, is dwarfed by the 48% by which solubility increased as atmospheric CO2 concentration rose by 48% (from 280 ppmv to 414 ppmv), and as atmospheric CO2 level continues to rise, the rate at which the oceans remove CO2 from the air will continue to accelerate.
For a more complete treatment of this issue, I strongly recommend this essay by Ferdinand Engelbeen:
http://www.ferdinand-engelbeen.be/klimaat/co2_origin.html
Additional Resources ↑
1. Here's a spreadsheet of CO2 emission data
from fossil fuels and cement manufacturing, along with measured atmospheric CO2 concentrations (from Mauna Loa and ice cores),
from 1750 to present. It's an exported Excel spreadsheet, which can be loaded directly by microsoft Excel. (For LibreOffice or OpenOffice
use the .xlsx file.)
2. Dr. Murry Salby has a long lecture on YouTube, arguing that nature, rather than mankind, is responsible for rising atomospheric CO2 levels. I critiqued it here.
3. The most thorough examination of the cause of rising CO2 concentration which I've found is this very clear and comprehensive analysis, by Ferdinand Engelbeen.
4. Nick Stokes also examines this topic, on his blog. (Don't overlook the comments.)
5. Takahashi, T et al (2002) analyzed hundreds of thousands of sea surface water samples, and found that the
influence of temperature on the equilibrium between ocean surface water and the atmosphere above it is about 4% per 1°C. They gave a concise formula for the relation:
∂ln pCO2/∂T=0.0423/K
or
pCO2(T) = pCO2(base) × EXP[0.0423 × (T - Tbase)]