Methane
Methane (CH4) is the second-most important anthropogenic greenhouse gas (GHG), after carbon dioxide (CO2). With methane and carbon dioxide at current levels, it is calculated by both MODTRAN Tropical Atmosphere and the NCAR Radiation Code that a 0.1 ppmv increase in atmospheric methane level has about the same warming effect as a 4.5 ppmv increase in CO2 level. (Some other authorities estimate more or less than that 45:1 ratio, e.g., the IPCC's AR5 Table 8.A.1 estimates 26.5:1.)
The main methane feedback mechanism is negative (attenuating), but methane is also involved in a widely discussed, mostly hypothetical, positive feedback process.
Atmospheric lifetime
Even if you don't burn it, methane in the atmosphere oxidizes fairly rapidly, changing ultimately into small amounts of CO2 and water: ↑
CH4 + 2⋅O2 → CO2 + 2⋅H2O (that's very simplified; see details [html] [2])
Various sources give the half-life of CH4 in the atmosphere as 6 to 8 years, which would make the average lifetime 1.4427 times that (because oxidation of methane is an exponential decay process), yielding an average lifetime for a molecule of CH4 in the atmosphere of 8.7 to 11.5 years. The AMS gives a figure of 9.1 years (from Pranther et al 2012, which actually reports a figure of 9.1±0.9 years). However, page 11 of this presentation by Prof. Lyatt Jaeglé gives the directly-calculated atmospheric lifetime of CH4 as ≈8 years, but identifies a feedback mechanism which she says effectively increases the atmospheric lifetime of additional CH4 to ≈12 years.
Call it 9-12 years (compared with about 50 years for CO2). That's pretty short. It means the only reason CH4 levels are as high as they are (about 1.9 ppmv† = 5.58 Gt) is that total CH4 emissions (natural + anthropogenic) are already high (between 580 and 710 Mt/yr). There would have to be a very large,‡ sustained increase in CH4 emissions to cause much increase in long-term average atmospheric CH4 levels.
Note that CH4 removal processes (mainly oxidation) dwarf the rate of CH4 accumulation in the atmosphere, and they accelerate with increasing atmospheric methane concentration, making them negative feedbacks. Assuming the Pranther atmospheric lifetime estimate (9.1±0.9 years), we can calculate that the rate of increase in CH4 level (which averages about 0.01 ppmv/year) is only about 1/60-th of the rate of the CH4 removal processes, and only about 1/35-th the rate of anthropogenic emissions. That means the CH4 level responds very quickly to changes in CH4 emission rate, and if the CH4 emission rate were to cease increasing then the level of CH4 in the atmosphere would rise at most only a few percent before plateauing.
Measurements
Methane levels have been monitored at Mauna Loa, Hawaii since 1983. During most of that time they've been inching up slightly, from about 1.65 ppmv to about 1.9 ppmv now. Here's a graph: ↑
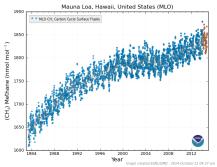
Click for full-sized, latest version
Ice core samples have extended the methane measurement record much further. Here's a smoothed graph of methane levels from 1840 to present:
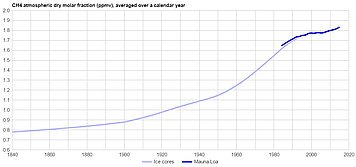
Click for full-sized, latest version
Radiative forcing, and warming effect
(Note: for a similar discussion w/r/t CO2 see: https://sealevel.info/Radiative_Forcing.html [or synopsis])
The radiative forcing from additional CH4 in the atmosphere can be determined though line-by-line spectral calculations, which, though daunting in their complexity, have been done most comprehensively by van Wijingaarden and Happer: ↑
● van Wijingaarden & Happer (2020), Dependence of Earth’s Thermal Radiation on Five Most Abundant Greenhouse Gases
This is Figure 5 from van Wijingaarden & Happer (2020); click here to enlarge it:
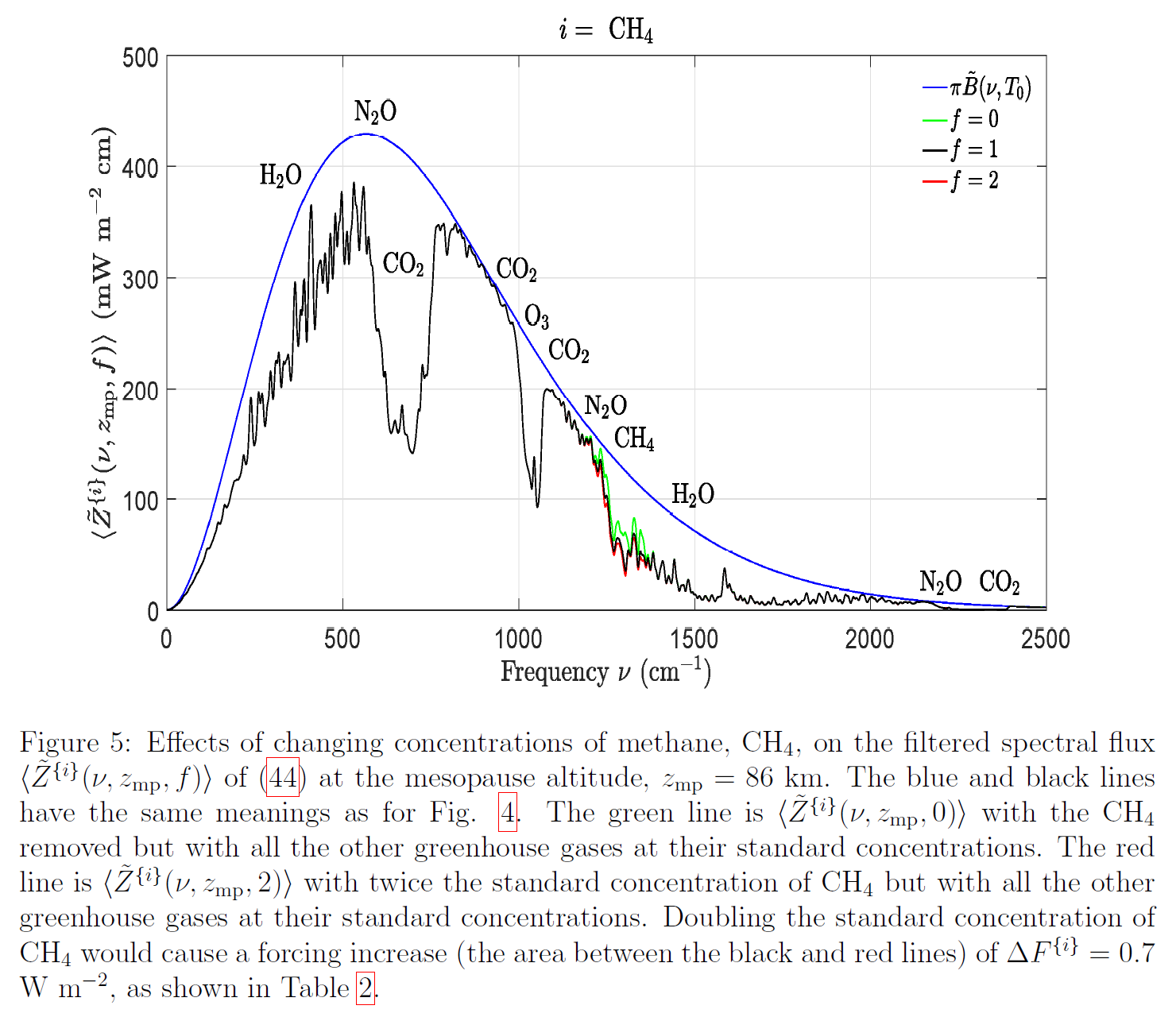
A doubling of CH4 concentration (from the current 1.9 ppmv to 3.8 ppmv) would cause a TOA radiative forcing of +0.7 W/m². Very roughly ⅓ °C of eventual warming can be expected for each 1 W/m² of radiative forcing, so doubling CH4 concentration would cause eventual (equilibrium) warming of about 0.2 °C.
Positive water vapor feedback might increase that slightly, but the overlap between the LW IR absorption spectrums of CH4 and H2O vapor reduces that amplification, so the possible net warming effect of a doubling of atmospheric CH4 concentration is certainly less than 0.3 °C.
†Methane levels vary slightly with measurement location; this site has some maps.
‡How large is “very large?” Well, for comparison, it would take about 3 Gt of CH4 to increase the atmospheric methane level by 1 ppmv.